

It may also be classified as a rare earth element.
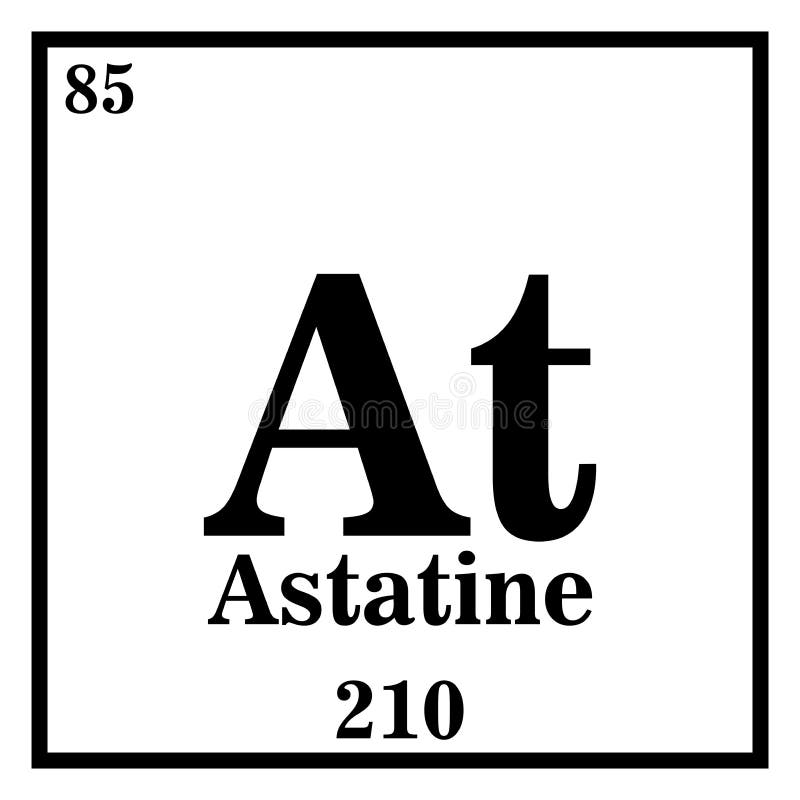
#Astatine symbol series
Americium is one of the elements in the actinide series of inner transition elements.
Symbol:"Am" Atomic Number:"95" Atomic Mass: (243)amu. You will also find aluminum in utensils, foil wrap, power lines, soda cans, and airplane structures. There is more aluminum than any other metal in the Earth's crust. Aluminum is a light element and classified as a basic metal. Symbol:"Al" Atomic Number:"13" Atomic Mass: 26.98amu. More Information: Chemical and Physical Changes in Matter In the middle ages, alchemists were people who tried to turn one element into another (usually lead (Pb) into gold (Au)). Some of the water molecules want to stick to you.Īlchemy is an ancient, non-scientific form of chemistry. You stay wet when you get out of the bathtub because of adhesive forces. More Information: Catalysts and InhibitorsĪdhesion is one type of attraction force between the molecules of a substance and the container or another object. Enzymes and catalysts can decrease the activation energy of a reaction. That required energy is the activation energy. If a reaction is not spontaneous, it requires a specific amount of energy to proceed. The energy required to start a chemical reaction. The energy needed to get the reaction started (get it over the hump) is called the activation energy. When reactions proceed, a certain amount of energy is needed for the whole process to begin. You will not find the element in regular use anywhere in the natural world.

It is used as a source of neutrons in experiments that involve radioactivity. Actinium is the first element of the actinide series. It is one of the elements in the actinide series of inner transition elements. Symbol:"Ac" Atomic Number:"89" Atomic Mass: 227.03amu. The elements include uranium, berkelium, and nobelium. Elements 89 through 103 are a part of this series. The actinide series is one of two series of inner transition elements. Think about those koosh balls for this example. This fact has key implications for the building up of the periodic table of elements.The absolute density is a measure of the mass of one milliliter of gas at standard temperature and pressure.Īn acicular habit describes the shape of a large crystal that looks like spikes coming out from one point.

The ordering of the electrons in the ground state of multielectron atoms, starts with the lowest energy state (ground state) and moves progressively from there up the energy scale until each of the atom’s electrons has been assigned a unique set of quantum numbers. It is the Pauli exclusion principle that requires the electrons in an atom to occupy different energy levels instead of them all condensing in the ground state. In the periodic table, the elements are listed in order of increasing atomic number Z. The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. The configuration of these electrons follows from the principles of quantum mechanics. The chemical properties of the atom are determined by the number of protons, in fact, by number and arrangement of electrons. See also: Atomic Number – Does it conserve in a nuclear reaction? Atomic Number and Chemical PropertiesĮvery solid, liquid, gas, and plasma is composed of neutral or ionized atoms. It is the electrons that are responsible for the chemical bavavior of atoms, and which identify the various chemical elements. In a neutral atom there are as many electrons as protons moving about nucleus. The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10 -19 coulombs. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z. The nucleus is composed of protons and neutrons. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.


 0 kommentar(er)
0 kommentar(er)
